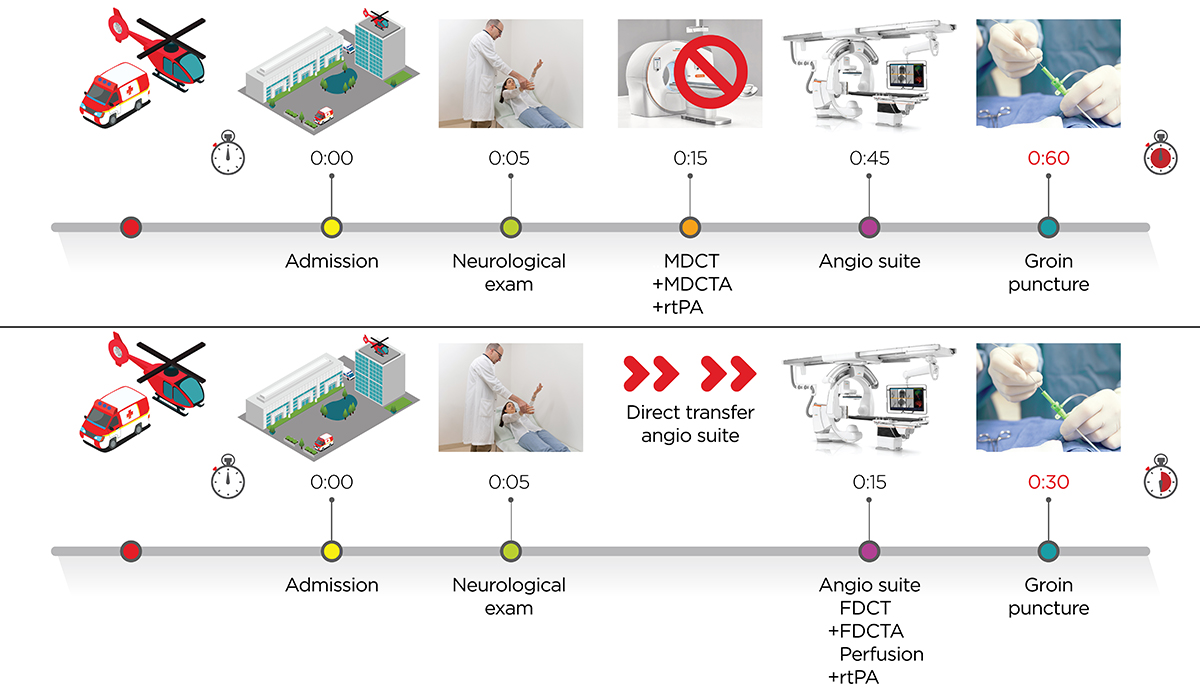
SIGNIFICANT REDUCTION OF DOOR-TO-GROIN TIMES
Several randomized trials have shown the superiority of endovascular stroke treatment compared to standard medical therapy.1-2 However, after this fundamental change in acute stroke patients’ care, several factors influencing the clinical outcome after stroke treatment came into focus.3 Continuous evolution of endovascular techniques resulting in higher revascularization rates and shorter intervention times are just one element in the vibrant development of endovascular stroke treatment.4-5
Probably even more important are the intrahospital time delays. A recently published meta-analysis focusing on intrahospital timings found every 4-minute delay in intrahospital time to result in 1/100 patients with a worse outcome on the modified Rankin Scale.6 The workflow analysis of the ESCAPE trial showed that the increase of every 30 minutes from CT imaging to reperfusion reduced the probability of achieving an independent functional outcome by 8.3%.7 Therefore, we and others focused on reducing intrahospital time delays and made substantial progress over the last years.8 Even after standardizing intrahospital procedures, door to groin times of around 60-70 min have been reported for direct admission patients.8, 9 From our perspective, stroke management has to rely on absolute necessary aspects of acute stroke care and abandon all unnecessary time delays occurring from admission to reperfusion. Hence, we developed and implemented a one stop management of stroke patients, in which the usual multidetector CT (MDCT) and MDCT-angiography/perfusion evaluation is bypassed. Patients presenting with a National Institutes of Health Stroke Scale (NIHSS) ≥ 7 (as of January 2017) are now directly transferred to the angio suite. They are then examined by flat detector CT (FDCT) and FDCT-angiography and treated by endovascular means in case of a large vessel occlusion (LVO) subsequently.
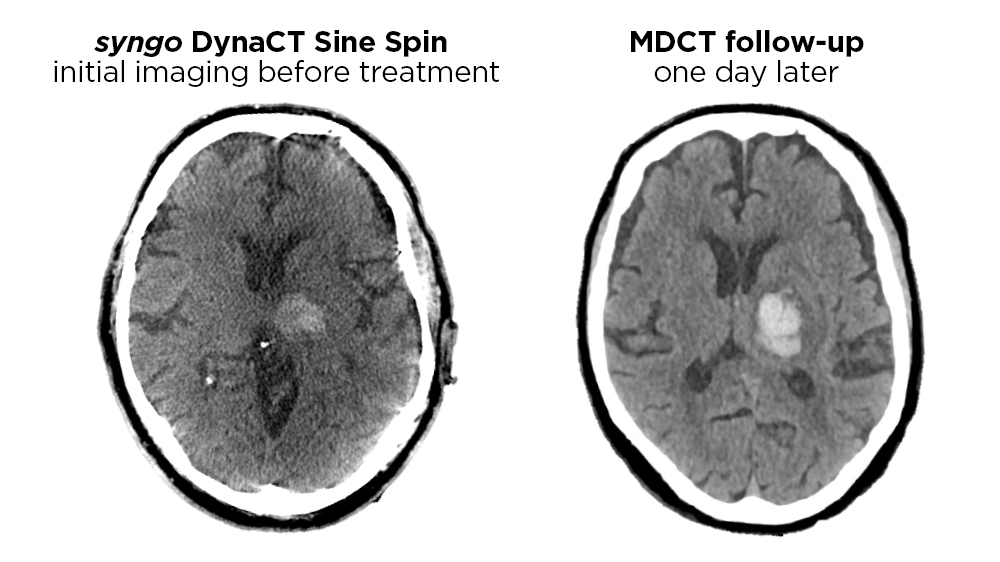
One of the biggest hurdles to a large-scale implementation of the one-stop-management approach so far has been the lack of clinical evidence to prove a reliable distinction between ischemic and hemorrhagic stroke by means of FDCT. Artifacts and decreased image quality near the base of the skull limited its use as triage tool in previous generations of angiography systems.
"ProSPective evaluation of the dIagnostic accuracy of siNe spiN non-contrast flat-dEtectoR CT (FDCT) for the detection of intracranial hemorrhage in Stroke patients"
The SPINNERS study is designed to evaluate whether FDCT imaging with syngo DynaCT Sine Spin has a comparable high diagnostic accuracy compared to MDCT in detecting intracranial.
Principal investigators of the SPINNERS study are Prof. Marios-Nikos Psychogios, Head of Diagnostic and Interventional Neuroradiology, University Hospital Basel and Dr. Adam S. Arthur, Chair of Neurosurgery at the University of Tennessee Health Sciences Center and Semmes-Murphey Clinic, Memphis, TN, US. The multi-center study is planned to be conducted at up to 12 clinical centers in the USA and Switzerland and is designed to include 252 patients.
The SPINNERS study is registered under ClinicalTrials.gov:

We first started our one stop management back in January 2016 with transfer patients. As the majority of those patients is treated with thrombolysis during transport to our comprehensive stroke center, repeated imaging is justified to exclude an intracranial hemorrhage. We recently published a paper showing high sensitivity and specificity of FDCT in the detection of intracranial hemorrhages.10 Based on this study, we also implemented this one stop management in direct admission or “mothership” patients in June 2016. 
We only included patients with an NIHSS ≥ 10 in this first step. After treating 30 patients with this protocol we analyzed our findings and have submitted a manuscript, which is currently under review. The initial experience shows that we were able to significantly reduce our door to CT and door to groin times, while successfully differentiating ischemic from hemorrhagic stroke. In January 2017, we lowered the one stop management threshold to an NIHSS ≥ 7 based on a study indicating that an NIHSS ≥ 7 in the best threshold for predicting LVO.11

Imaging Protocol
Patients presenting with neurological symptoms consistent with a severe acute ischemic stroke (according to a score of 7 and above on the National Institutes of Health Stroke Scale) are directly transferred to our angiography suite (ARTIS icono; Siemens Healthcare GmbH, Forchheim, Germany). Our one stop approach consists of a non-contrast Sine Spin FDCT scan, a portrait FDCT angiography (FDCT-A) and a FDCT perfusion (FDCT-P). The following parameters are applied for the non-contrast Sine Spin FDCT, the portrait FDCT-A and the FDCT-P respectively:
Non-contrast Sine Spin FDCT – 7s rotation; 220° total angle with approx. 550 projections; 109 kV; 1.8 μGy/frame; effective dose ∼2 mSv12
Portrait FDCT-A - intravenous injection of 60 ml contrast agent (Imeron 400; Bracco Imaging GmbH, Konstanz, Germany) at an injection rate of 5 ml/s followed by 60 ml saline chaser at the same injection rate of 5 ml/s; a power injector is used for injection; 1 x 10 s rotation, 200° total angle (0.8° per frame), 90 kV, 1,2 μGy/frame; effective dose ∼0.91 mSv.
FDCT-P - intravenous injection of 60 ml contrast agent (Imeron 400; Bracco Imaging GmbH, Konstanz, Germany) at an injection rate of 5 ml/s followed by 60 ml saline chaser at the same injection rate of 5 ml/s; a power injector is used for injection; 10 x 5 s rotation, 200° total angle (0.8° per frame), 70 kV, 0,36 μGy/frame; effective dose ∼2.88 mSv (collimated).
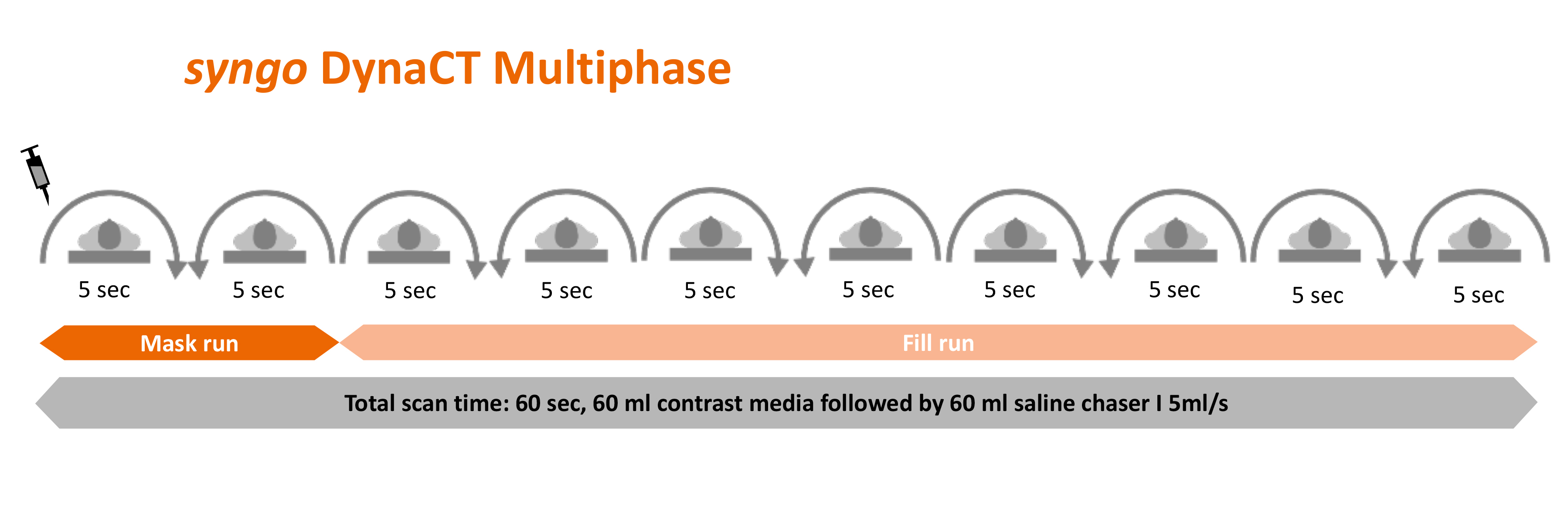
For the non-contrast Sine Spin FDCT, a “HU smooth” kernel and “DynaCT Clear” algorithm are applied to obtain images with a 512 × 512 matrix. These are reconstructed with 5 mm slice thickness and 3 mm interslice distance. FDCT images are used for the detection of intracranial hemorrhages and large ischemic lesions. Images acquired according to our protocol proved to be a reliable and accurate tool for the detection of intracranial hemorrhage. Gray–white differentiation is feasible in the supratentorial region.10, 12
The rotation of the portrait FDCT-A is timed after a bolus-tracking digital subtraction angiography to capture the peak arterial phase. The portrait FDCT-A has an increased field of view compared to the traditional “landscape” FDCT-A due to a rotation of the detector by 90° (see figure X for comparison). It enables the physician to simultaneous visualize the circulus willisii and the intra- and extracranial carotid arteries down to their origin. This information is crucial for the planning of the intervention. For reconstruction of the FDCT-A, a “HU normal” kernel is utilized. Images are reconstructed as maximum intensity projections (MIPs) with a slice thickness of 24 mm and an interslice distance of 3 mm. It allows for the reliable detection of large vessel occlusions.


We collimate the FDCT-P for depiction of the cerebrum only, thus reducing the radiation dose substantially (almost 50%). The FDCT-P images are automatically reconstructed using RAPID AI for Angio (see figure X). As a recent publication showed a strong correlation between FDCT-P and MDCT-P for the automated measurements of core and mismatch values, this protocol can be used for patient selection.13 Recent studies showed that Perfusion imaging has a higher sensitivity for the detection of distal vessel occlusions.14 We therefore use this protocol also in the early time-window to detect patients suitable for endovascular therapy. This approach ensures that no patient with a distal but treatable vessel occlusion is missed.
The effective dose of our stroke protocol (overall effective dose of ~5.5 mSv) is comparable to published MDCT stroke protocols with perfusion imaging. Although the coverage of our FDCT-A protocol is not equal to cervical MDCT-A scans depicting the aortic arch, the coverage is long enough to evaluate the internal and external carotid arteries down to their origins.
References
- Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine 2015;372:11-20
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-1731
- Jovin TG, Albers GW, Liebeskind DS. Stroke Treatment Academic Industry Roundtable: The Next Generation of Endovascular Trials. Stroke; a journal of cerebral circulation 2016;47:2656-2665
- Behme D, Knauth M, Psychogios MN. Retriever wire supported carotid artery revascularization (ReWiSed CARe) in acute ischemic stroke with underlying tandem occlusion caused by an internal carotid artery dissection: Technical note. Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences 2017:1591019917690916
- Maus V, Behme D, Kabbasch C, et al. Maximizing First-Pass Complete Reperfusion with SAVE. Clinical Neuroradiology 2017:1-12
- Saver JL, Goyal M, van der Lugt A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. Jama 2016;316:1279-1288
- Menon BK, Sajobi TT, Zhang Y, et al. Analysis of Workflow and Time to Treatment on Thrombectomy Outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) Randomized, Controlled Trial. Circulation 2016;133:2279-2286
- Schregel K, Behme D, Tsogkas I, et al. Effects of Workflow Optimization in Endovascularly Treated Stroke Patients - A Pre-Post Effectiveness Study. PloS one 2016;11:e0169192
- Frei D, McGraw C, McCarthy K, et al. A standardized neurointerventional thrombectomy protocol leads to faster recanalization times. Journal of NeuroInterventional Surgery 2016
- Leyhe JR, Tsogkas I, Hesse AC, et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. Journal of NeuroInterventional Surgery 2016
- Heldner MR, Hsieh K, Broeg-Morvay A, et al. Clinical prediction of large vessel occlusion in anterior circulation stroke: mission impossible? Journal of neurology 2016;263:1633-1640
- Petroulia VD, Kaesmacher J, Piechowiak EI, et al. Evaluation of Sine Spin flat detector CT imaging compared with multidetector CT. Journal of NeuroInterventional Surgery 2022
- Quispe-Orozco D, Farooqui M, Zevallos C, et al. Angiography Suite Cone-Beam Computed Tomography Perfusion Imaging in Large-Vessel Occlusion Patients Using RAPID Software: A Pilot Study. Stroke 2021;52(9):e542-e4.
- Amukotuwa SA, Wu A, Zhou K, et al. Distal Medium Vessel Occlusions Can Be Accurately and Rapidly Detected Using Tmax Maps. Stroke 2021;52(10):3308-17.

